|
Because of the small NMR signal generated from ¹³C, much larger voxels and/or longer imaging times are required. Special techniques, such as proton decoupling, Nuclear Overhauser Enhancement (NOE), and hyperpolarization methods are needed to boost the weak signal. Infusion of ¹³C-enriched metabolic substrates such as glucose prior to imaging may also be employed.
|
Advanced Discussion (show/hide)»
The resonance frequency of ¹³C is only about one-fourth as large as ¹H, so dual tuned coils and dedicate hardware are required.
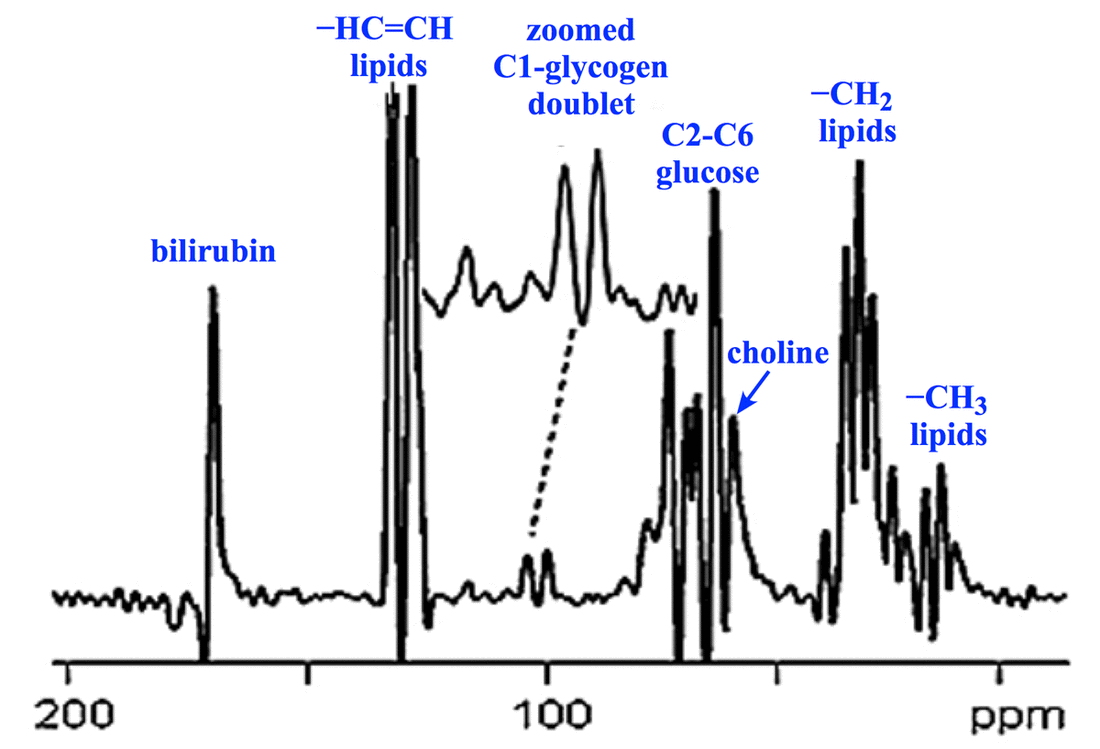
Chemical shifts for ¹³C extend over a much wider range than for ¹H, typically from 0 to over 200 ppm. This makes recognition of peaks much more straightforward than in ¹H spectroscopy. Like ¹H MRS, tetramethylsilane (TMS) also serves as the δ = 0 ppm reference point for carbon spectroscopy.
The observed ¹³C chemical shifts reflect in part the hybridization state (sp, sp², sp³) of each nucleus. Values roughly parallel those seen in ¹H spectroscopy: alkanes (10 – 40 ppm), alkenes (40 – 70 ppm), aromatics (115 – 150 ppm), and carboxy-acids (160 – 185 ppm).
Heteronuclear J-coupling by adjacent ¹H nuclei is a prominent feature of carbon spectroscopy, with one bond C−H coupling constants in the range of 100 – 250 Hz. Splitting of carbon peaks (via the n+1 rule) allows us to predict the number of ¹H nuclei attached to a given carbon.
Because of ¹³C’s low natural abundance (1.1%), C−C coupling is not observed because the chance is that two ¹³C nuclei would occur immediately adjacent to one another on a given molecule is very small.
Quantifying carbon NMR spectra is also difficult in that areas under carbon peaks does not accurately reflect the relative numbers of carbon atoms contained. Carbons with more ¹H nuclei attached will have inherently higher signals than those with few or no ¹H nuclei attached.
Carbon-13 nuclear magnetic resonance. Wikipedia, the Free Encyclopedia, accessed 2017.
Golman K, Olsson LE, Axelsson O, et al. Molecular imaging using hyperpolarized ¹³C. Br J Radiol 2003; 76:S118-127.
Gruetter R, Adriany G, Choi I-Y, et al. Localized in vivo ¹³C NMR spectroscopy of the brain. NMR Biomed 2003; 16:313-338.
Hwang J-H, Choi CS. Use of in vivo magnetic resonance spectroscopy for studying metabolic diseases. Exp Mol Med 2015; 47, e139.
Ross B, Lin A, Harris K, et al. Clinical experience with ¹³C MRS in vivo. NMR Biomed 2003; 16:358-369.
Wang ZJ, Ohliger MA, Larson PEZ, et al. Hyperpolarized ¹³C MRI: State of the art and future directions. Radiology 2019; 291:273-284.
What nuclei besides hydrogen can be investigated with MRS?
What is NOE? How and when should it be used for phosphorus MRS?
What is decoupling? Is it required for phosphorus spectroscopy?